本帖最后由 老马 于 2012-1-13 21:20 编辑
: m5 u2 d/ C, `, r3 U
/ m4 r: Y3 a6 v" q5 k: a L爱必妥和阿瓦斯丁的比较
# w& b6 n) Z# R
 + {+ C1 s4 v# X" C
+ {+ C1 s4 v# X" C
http://cancergrace.org/lung/2008/08/30/bms099-os-neg/: J: T/ C8 R0 `9 ?/ {% p$ C# p
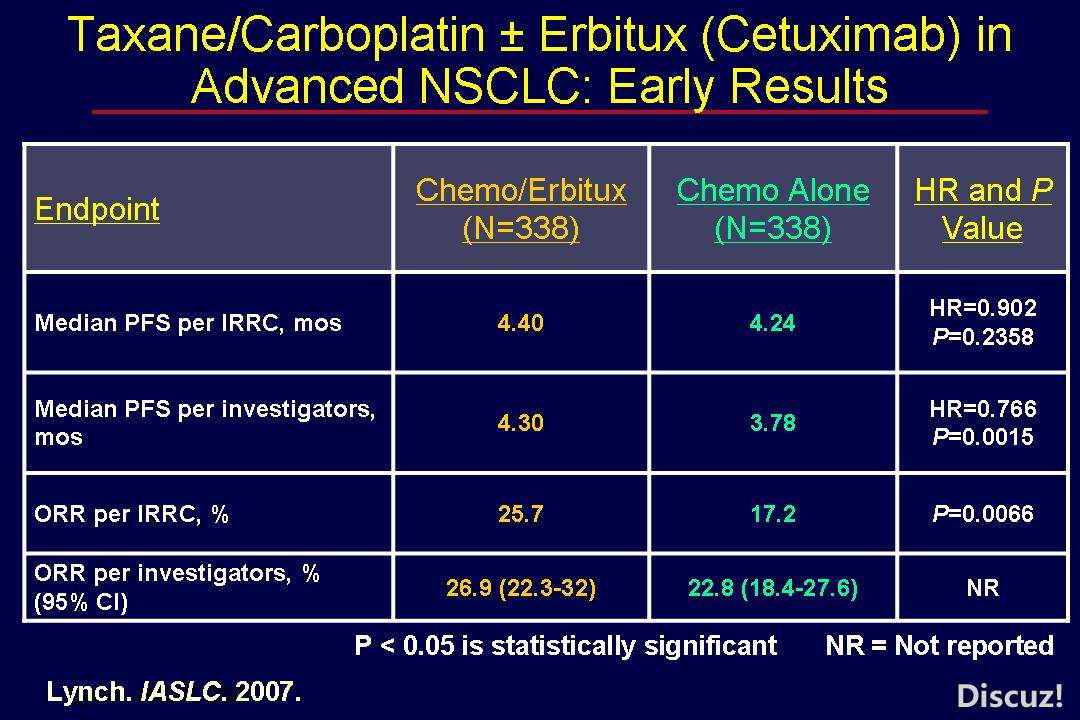
7 h, t( }* X0 L0 G
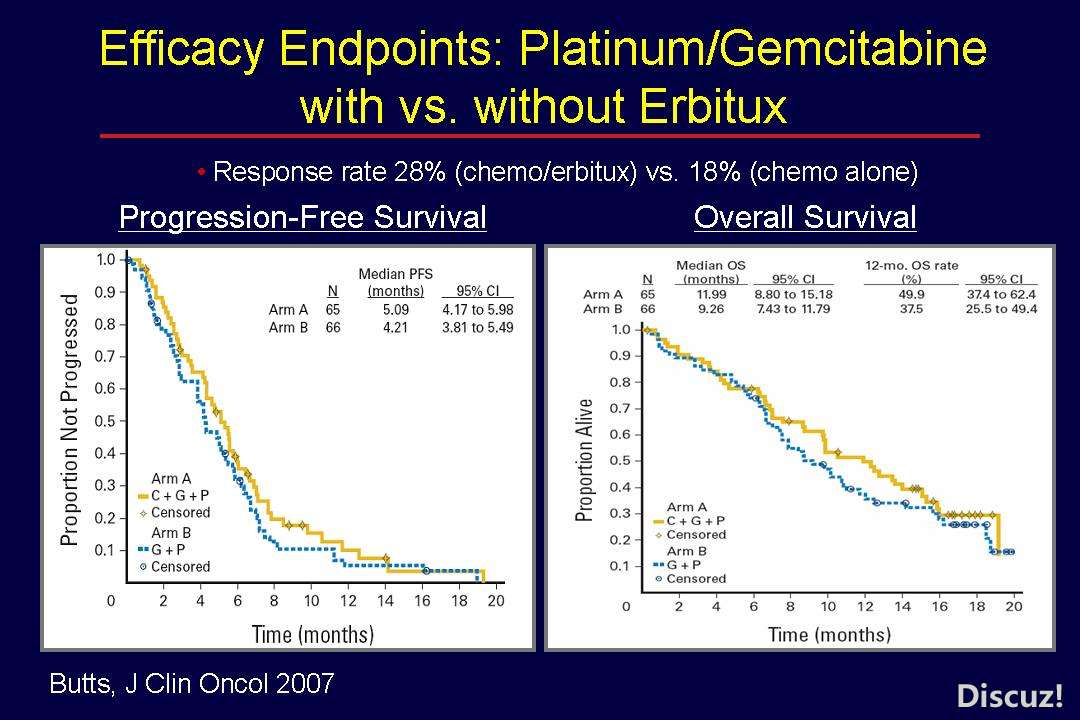 6 c5 Q: I! s! N) J6 z
6 c5 Q: I! s! N) J6 z
http://cancergrace.org/lung/2007/12/27/platgem-erbitux-trial/
: J* ~- Y/ {$ W) J% Y% V( E==================================================
' G2 q; G9 ^- K+ X2 qOverall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL)
& s+ O$ k9 k' t0 S+ [Patients and methods: Patients (n = 1043) received cisplatin 80 mg/m2 and gemcitabine 1250 mg/m2 for up to six cycles plus bevacizumab 7.5 mg/kg (n = 345), bevacizumab 15 mg/kg (n = 351) or placebo (n = 347) every 3 weeks until progression. Primary end point was progression-free survival (PFS); OS was a secondary end point.
# K G- m4 N0 l* _' `8 s; \4 b. \Results: Significant PFS prolongation with bevacizumab compared with placebo was maintained with longer follow-up {hazard ratio (HR) [95% confidence interval (CI)] 0.75 (0.64–0.87), P = 0.0003 and 0.85 (0.73–1.00), P = 0.0456} for the 7.5 and 15 mg/kg groups, respectively. Median OS was >13 months in all treatment groups; nevertheless, OS was not significantly increased with bevacizumab [HR (95% CI) 0.93 (0.78–1.11), P = 0.420 and 1.03 (0.86–1.23), P = 0.761] for the 7.5 and 15 mg/kg groups, respectively, versus placebo. Most patients (~62%) received multiple lines of poststudy treatment. Updated safety results are consistent with those previously reported.
# h# I! V; N, y' f) Q# e

|